Vaccination
Introduction
Vaccines are produced from a part or all of the structure of viruses or bacteria that are killed or weakened from subunit proteins in the virus
Antigens in vaccines are able to stimulate the immune system in the body
Vaccines help the immune system identify specific germs so that the immune system is well prepared to be attacked by real germs
Importance taking Vaccine
According to the source,the Covid-19 vaccine is given to control the spread of Covid-19 disease. As more and more people get vaccinated, many people will form stronger antibodies to reduce severe complications when infected by Covid-19 disease.Indirectly, it can not only protect the risky.In fact, everyone including children and the elderly can be protected.
Free for all people
As humanitarian measures
Effects
Studies show vaccination takes one and two weeks to elicit an immune response
Type of Vaccine
mRNA - pFIZER
Vector
Inactivated
Virus Like Particle / VLP
DNA
Life Attenuated-Sinovac
Viral Sub Unit-Astra Zenecca, Sputnik
- 1) Vaccines include: Sinopharm, Sinovac
- Number of doses required: 2 doses, intramuscular
- Other licensed vaccines that use this type of technology: Hepatitis A, polio, rabies (all inactivated type).
- What to know: The whole virus vaccine uses a weakened or deactivated form of the pathogen that causes COVID-19 to trigger protective immunity to it.
- The two vaccines mentioned above call as Sinopharm and Sinovac were both use inactivated pathogens, therefore they cannot infect cells and replicate, but can trigger an immune response.
- Benefits: According to Gavi, the Vaccine Alliance (GAVI), the advantages of an inactivated whole virus vaccine include the fact its technology is well established, it is suitable for people with compromised immune systems, and it is relatively simple to manufacture.
- Challenges: Booster shots may be required.

Vaccine Sinopharm
Vaccine Sinovac
- 3) NON-REPLICATING VIRAL VECTORVaccines include: Oxford-AstraZeneca, Sputnik V (Gamaleya Research Institute)
- Number of doses required: 2 doses, intramuscular
- Other licensed vaccines that use this type of technology: Ebola
- What to know: This type of vaccine introduces a safe and modified version of the virus that were known as the vector that have functions to deliver genetic code for the antigen. In a COVID-19 vaccine the vector is the spike proteins found on the surface of the coronavirus.
- Once the body cells are infected, the cells are instructed to produce a large amount of antigens which in turn trigger an immune response.
- Benefits: Viral vector based vaccination is another well established technology that can trigger a strong immune response as it also involves both B cells and T cells.
- Challenges: Previous exposure to the vector could reduce effectiveness, plus these types of vaccine is relatively complex to manufacture compared to others.
Oxford-AstraZeneca
Sputnik V
- 4) PROTEIN SUBUNIT
- Vaccines include: Novavax
- Number of doses required: 2 doses, intramuscular
- Other licensed vaccines that use this type of technology:Hepatitis B, meningococcal disease, pneumococcal disease, shingles
- What to know: The protein subunit vaccine contains purified pieces of a pathogen rather than the whole pathogen to trigger an immune response. It is thought that by restricting the immune system to the whole pathogen, the risk of side effects is minimised.
- Benefits: The protein subunit vaccination is also a well-established technology that is advantageous for those with compromised immune system.
- Challenges: This type of vaccine is relatively complex to manufacture, and adjuvants and booster shots may be required.
Novavax
Why are there so many vaccines in development?
Typically many vaccine candidates will be evaluated before any are found to be both safe and effective. For example, of all the vaccines that are studied in the lab and laboratory animals roughly 7 out of every 100 will be considered good enough to move into clinical trials in humans. Of the vaccines that do make it to clinical trials, just one in five is successful. Having lots of different vaccines in development increases the chances that there will be one or more successful vaccines that will be shown to be safe and efficacious for the intended prioritized populations.
Development of Vaccine
The different types of vaccines
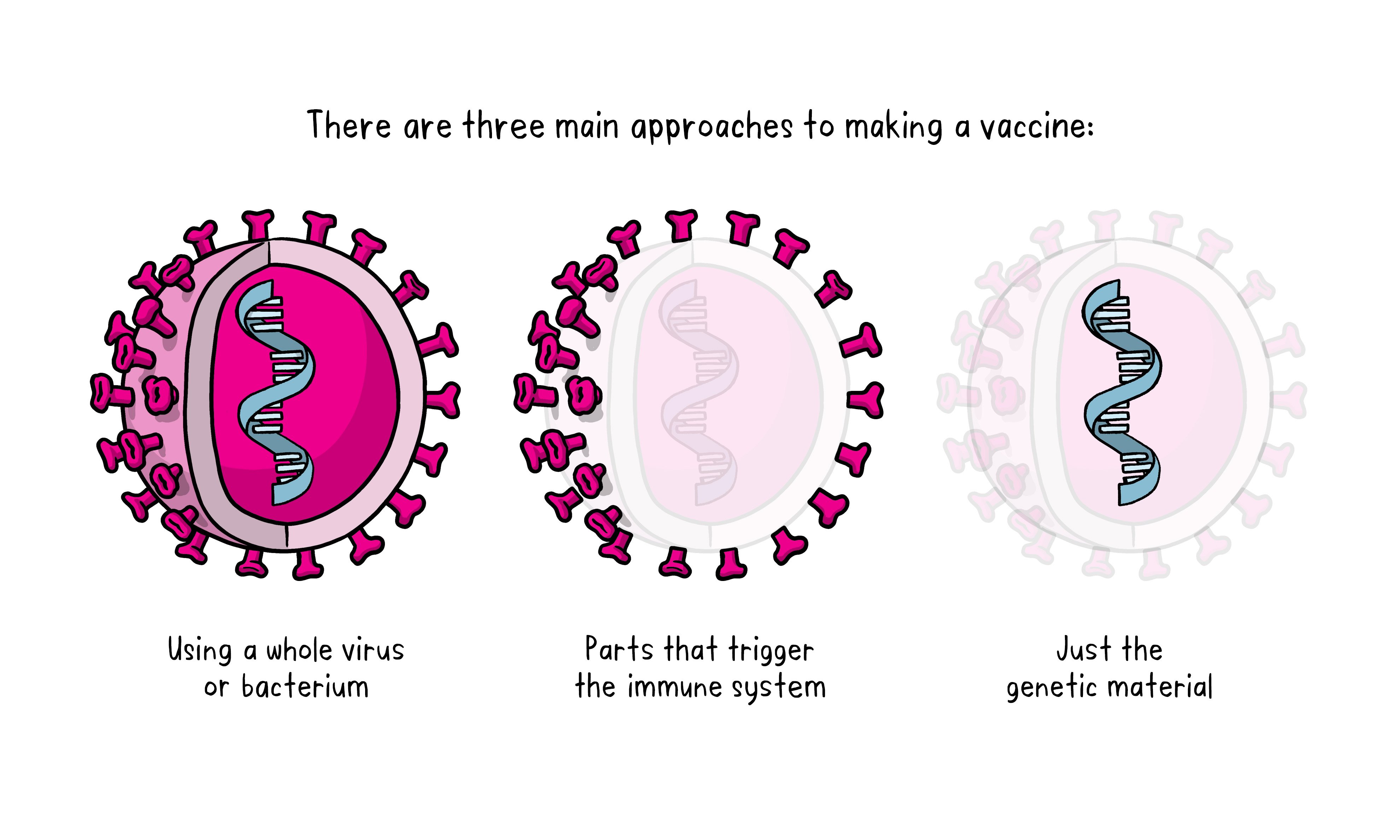
There are three main approaches to designing a vaccine. Their differences lie in whether they use a whole virus or bacterium just the parts of the germ that triggers the immune system or just the genetic material that provides the instructions for making specific proteins and not the whole virus.
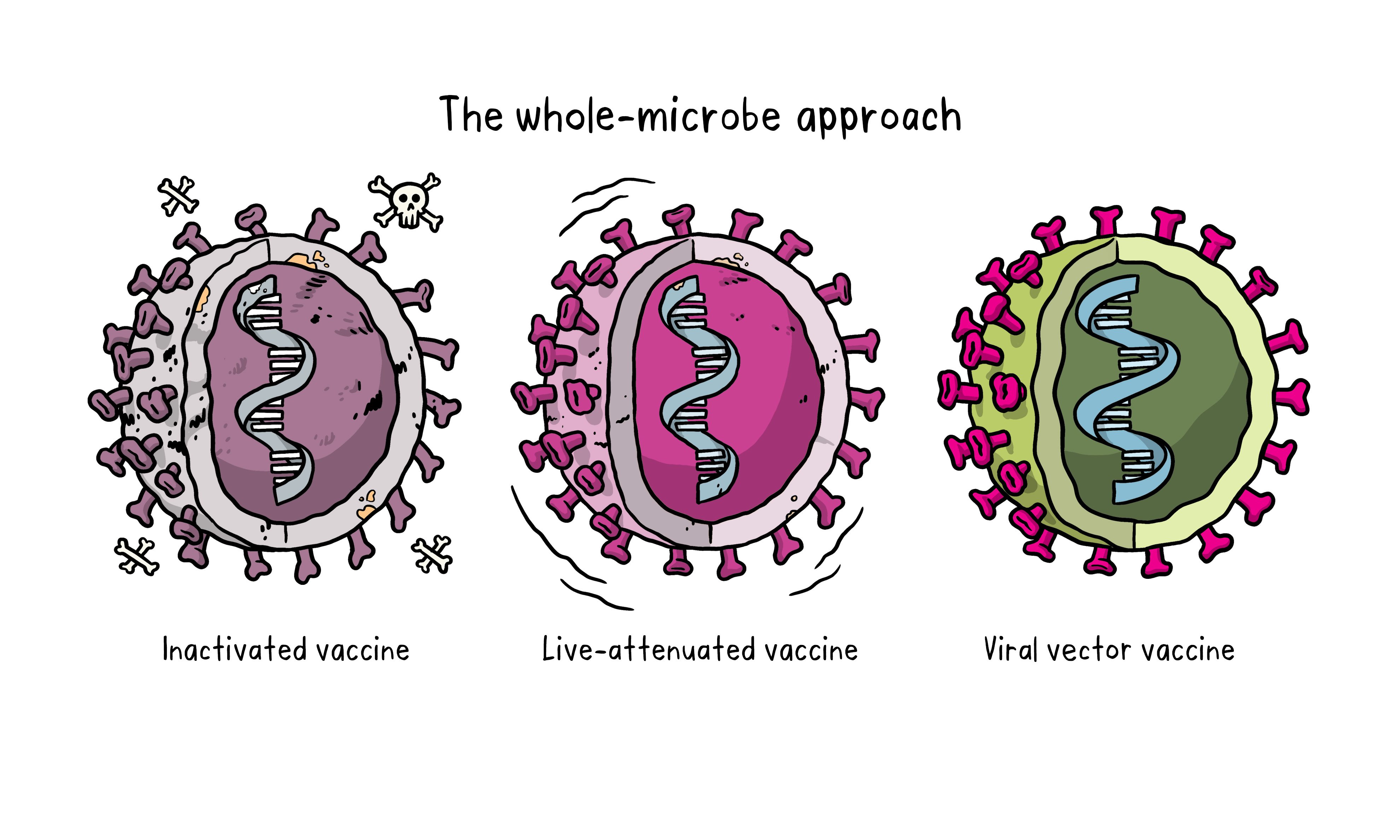
Inactivated Vaccine
Inactivated vaccine
The first way to make a vaccine is to take the disease-carrying virus or bacterium, or one very similar to it, and inactivate or kill it using chemicals, heat or radiation. This approach uses technology that is been proven to work in people. This is the way the flu and polio vaccines are made and vaccines can be manufactured on a reasonable scale. However, it requires special laboratory facilities to grow the virus or bacterium safely, can have a relatively long production time, and will likely require two or three doses to be administered.
Live-attenuated vaccine
A live-attenuated vaccine uses a living but weakened version of the virus or one that is very similar. The measles, mumps and rubella (MMR) vaccine and the chickenpox and shingles vaccine are examples of this type of vaccine. This approach uses similar technology to the inactivated vaccine and can be manufactured at scale. However, vaccines like this may not be suitable for people with compromised immune systems.
Viral vector vaccine
This type of vaccine uses a safe virus to deliver specific sub-parts called proteins of the germ of interest so that it can trigger an immune response without causing disease. To do this, the instructions for making particular parts of the pathogen of interest are inserted into a safe virus. The safe virus then serves as a platform or vector to deliver the protein into the body. The protein triggers the immune response. The Ebola vaccine is a viral vector vaccine and this type can be developed rapidly.
The subunit approach
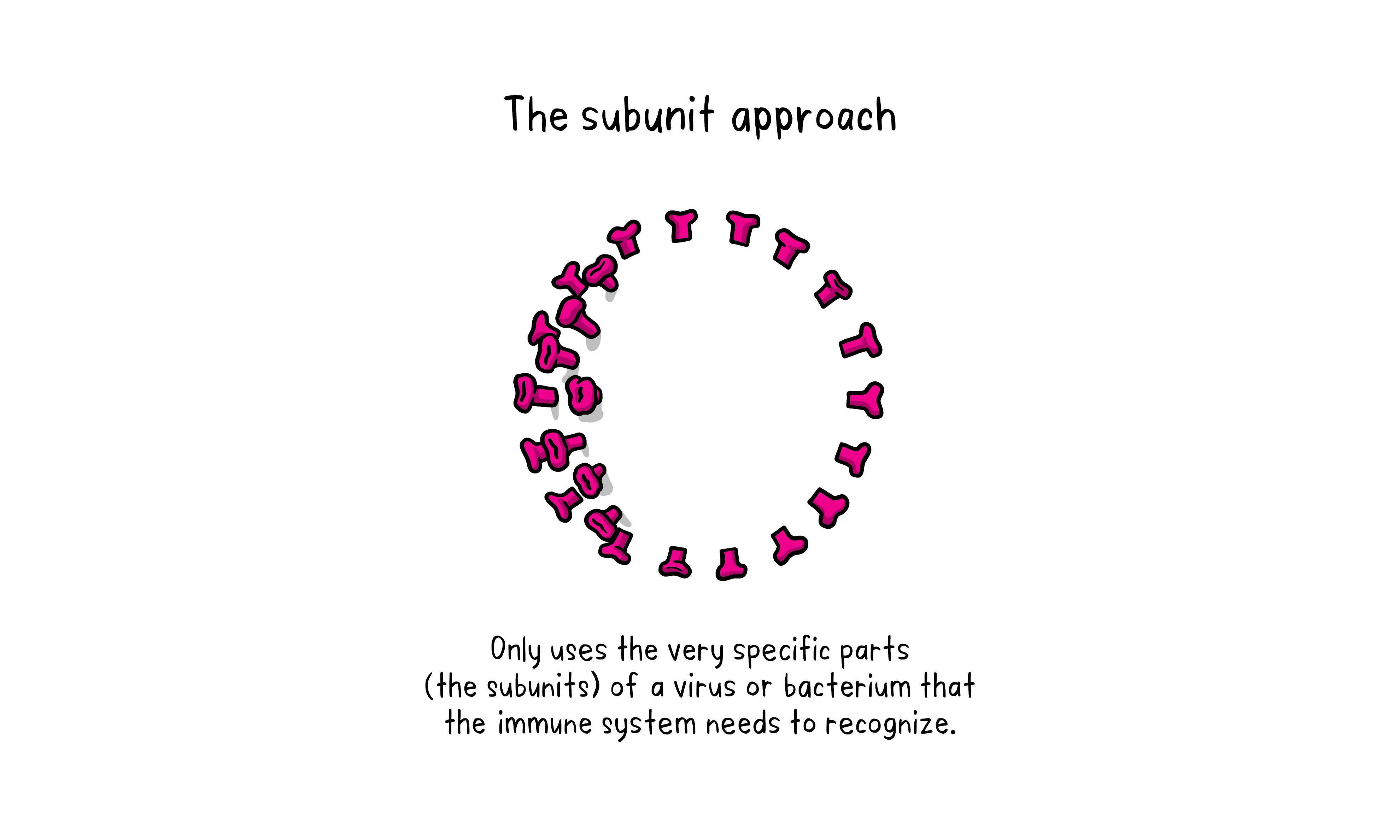
Subunit Approach
A subunit vaccine is one that only uses the very specific parts (the subunits) of a virus or bacterium that the immune system needs to recognize. It does not contain the whole microbe or use a safe virus as a vector. The subunits may be proteins or sugars. Most of the vaccines on the childhood schedule are subunit vaccines protecting people from diseases such as whooping cough, tetanus, diphtheria and meningococcal meningitis.

Genetic Approach
The genetic approach (nucleic acid vaccine)
Unlike vaccine approaches that use either a weakened or dead whole microbe or parts of one, a nucleic acid vaccine just uses a section of genetic material that provides the instructions for specific proteins, not the whole microbe. DNA and RNA are the instructions our cells use to make proteins. In our cells, DNA is first turned into messenger RNA, which is then used as the blueprint to make specific proteins.
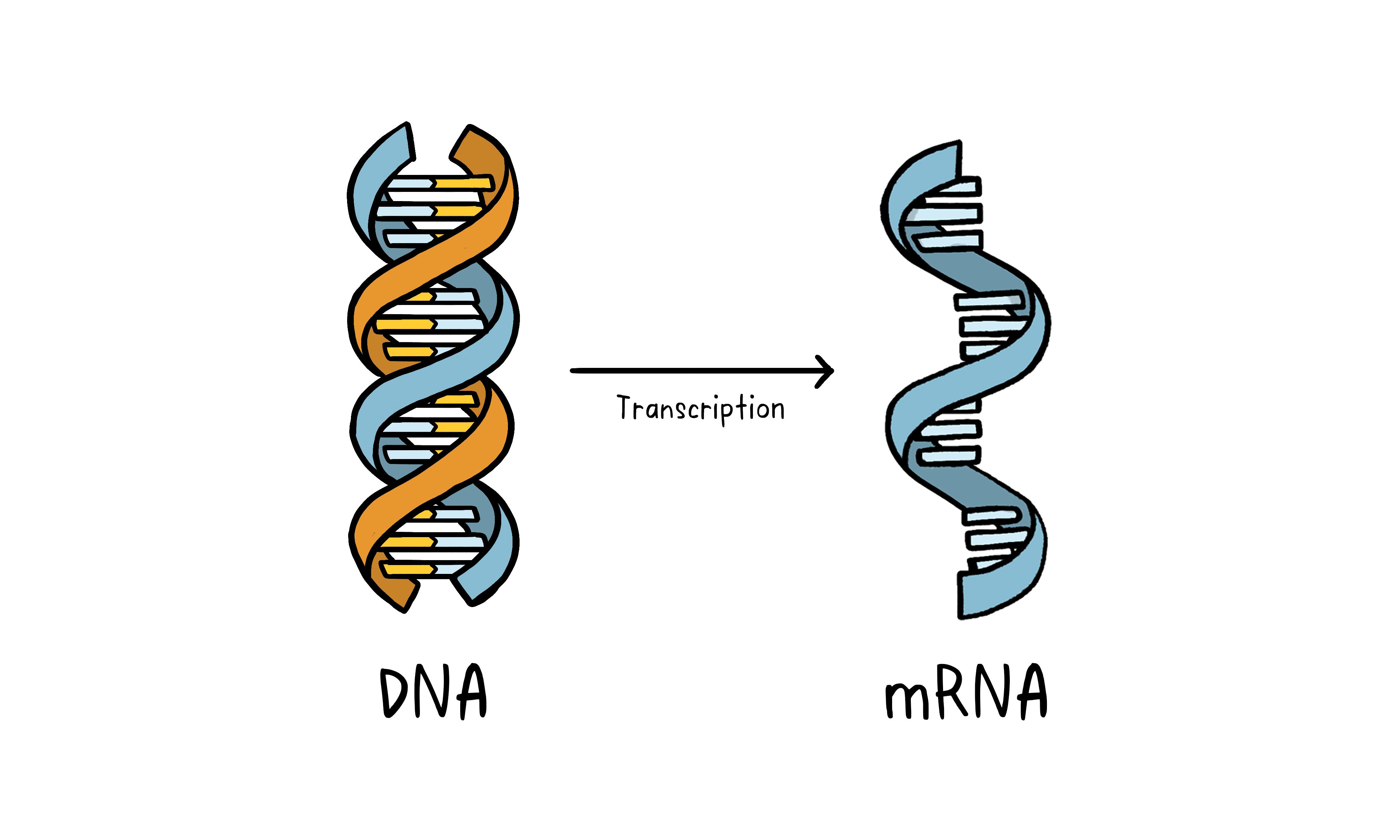
DNA and mRNA
A nucleic acid vaccine delivers a specific set of instructions to our cells, either as DNA or mRNA, for them to make the specific protein that we want our immune system to recognize and respond to. The nucleic acid approach is a new way of developing vaccines. Before the COVID-19 pandemic, none had yet been through the full approvals process for use in humans, though some DNA vaccines, including for particular cancers, were undergoing human trials. Because of the pandemic, research in this area has progressed very fast and some mRNA vaccines for COVID-19 are getting emergency use authorization, which means they can now be given to people beyond using them only in clinical trials.